
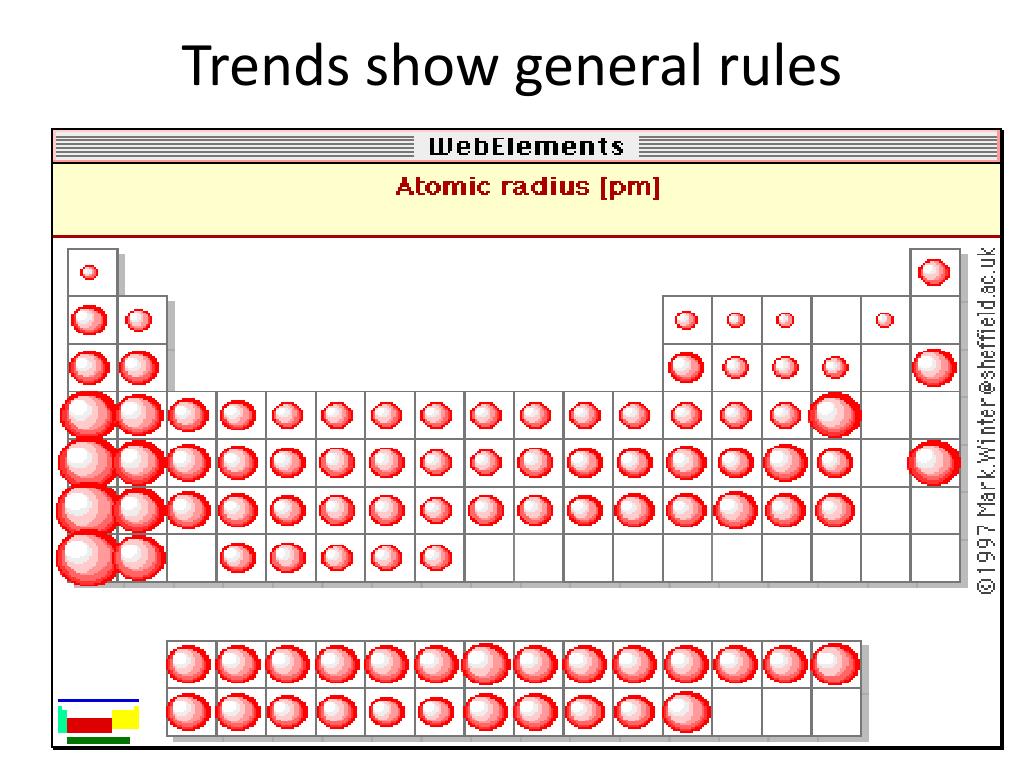
On the other hand, the larger elements, i.e. nitrogen, fluorine, oxygen, TEND to be very powerful oxidants, and this is also manifested by their small atomic size. Excluding the Noble Gases, the smaller atoms from the right hand side, i.e. Because of the way we organize the elements, there are special patter. It follows that the SMALLEST atoms derive the right of the Table as we face it. Why is the periodic table arranged the way it is There are specific reasons, you know.
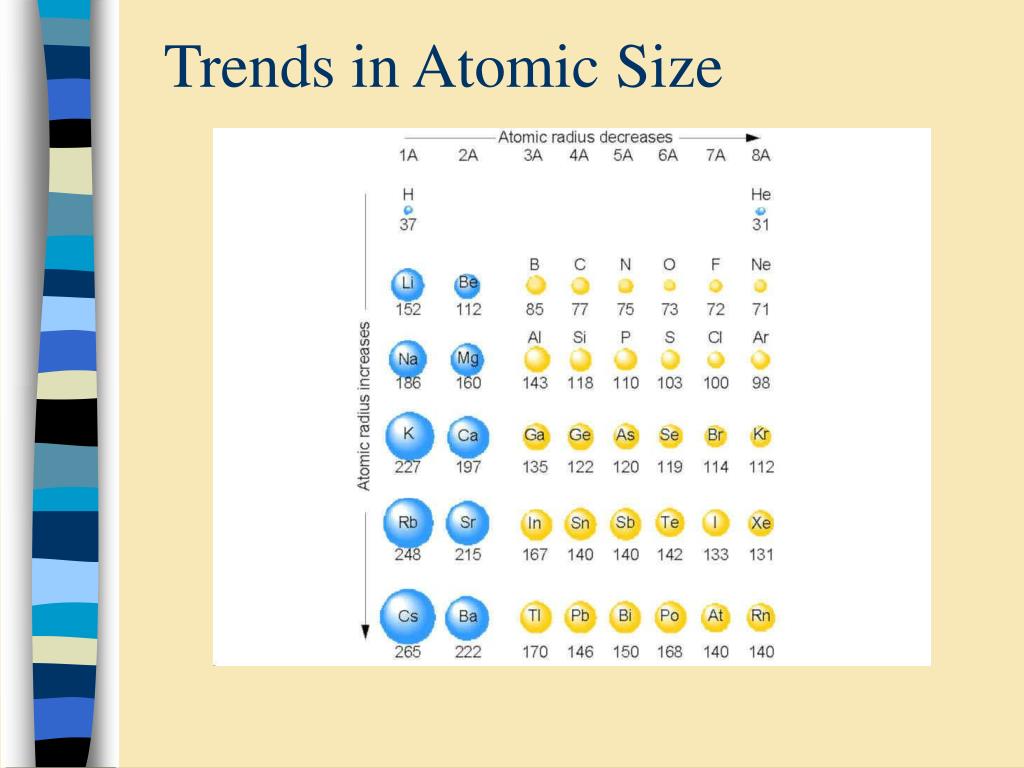
Of course, the diagram shows NO data (it should do so), but the relative size of the atoms across the Period, and down the Group is clear. Compare electron affinities and electronegativities. And the best metric that illustrates this trend is the well-known diminution of atomic radii across the Period from left to right? And of course, we should look at some data. Predict greater or smaller atomic size and radial distribution in neutral atoms and ions. Now it is a fact that the nuclear charge is SHIELDED very poorly by incomplete electronic shells. The chemistry and atomic structure of the elements is a contest between (i) nuclear charge, conveniently represented by #Z_"the atomic number"#, and (ii) shielding by other electrons. Since 0.85 Å is the only option that is less than 1.06 Å, the correct option is 'd'.#"Increase in atomic radii down a Group, a column of the Periodic"#"Table."# In lanthanoids, the atomic and ionic radii decrease with increase in atomic number. Lanthanum is the first element and Lutetium is the last one.

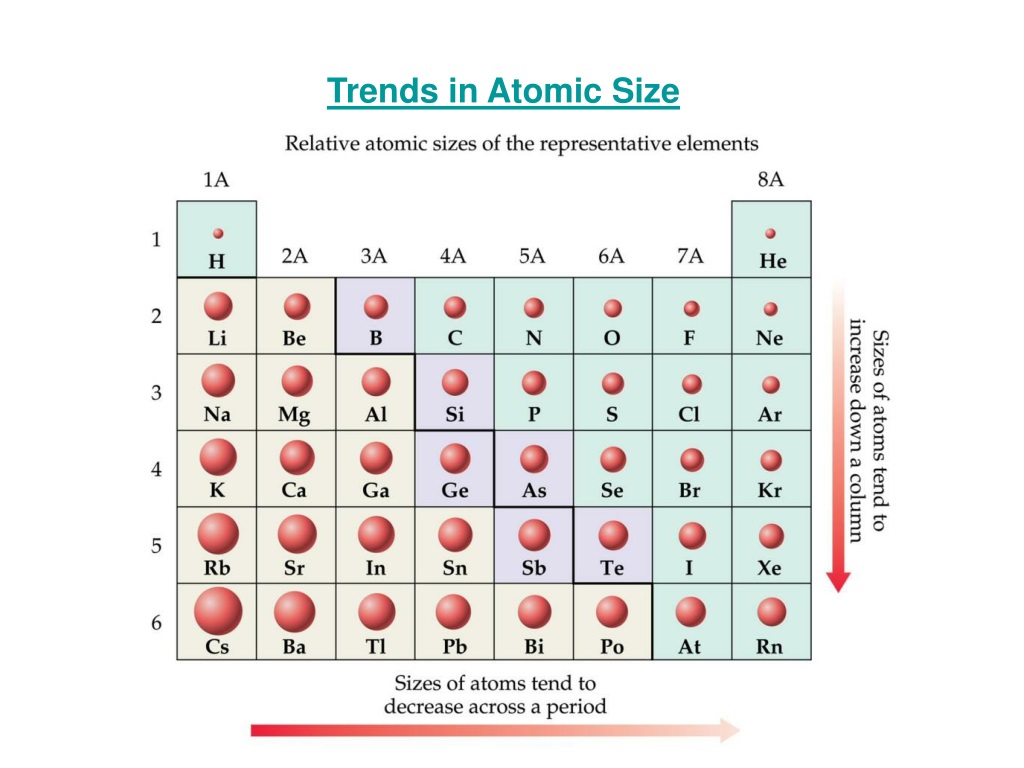
Lanthanum, La and Lutetium, Lu belong to lanthanoids (4f block elements of inner transition elements). Which one of the following given values will be closest to the radius of Lu 3+ (atomic number : Lu =71) ? 6) The radius of La 3+ (atomic number : La=57) is 1.06 Å. The correct order of ionic radii is Na + > Mg 2+ > Al 3+ > Si 4+ as given in option "4". The correct option is "d" 5) Which of the following is correct order of ionic radii?įor isoelectronic ions, the radius decreases with increase in positive charge. For different ions of same element, size decreases with increase in the positive charge. K + and Cl - are isoelectronic species but the number of protons are more in K +. Since the proton number is same in isotopes, the nuclear attraction is also same. (Credit: Christopher Auyeung Source: CK-12 Foundation License: CC BY-NC 3. 2: The atomic radius (r) ( r) of an atom can be defined as one half the distance (d) ( d) between two nuclei in a diatomic molecule. These ions belong to two different isotopes of same element. The atomic radius is defined as one-half the distance between the nuclei of identical atoms that are bonded together. The size decrease with increase in the positive charge these species. The atomic size increases from top to bottom in a group. All other elements have an atomic radius lying between these 2 values. With the exception of hydrogen (with an atomic radius of 0.11. Size decreases with increase in proton number Atomic radii The size of atoms is usually best described by their radius. The trend in atomic radius across the periodic table also varies from the trend in ionic radius. The size of ions is either greater or lesser than the parent atom. The ionic radii increases with decrease in the effective nuclear charge. No, the atoms are electrically neutral substances, while ions have electrons less or more than the parent atom in order to attain stability. Generalization: In iso-electronic species, the atomic size decreases with increase in the atomic number. Hence the attraction is minimum and the ion is largest. However, in N 3- ion, there is only 0.7 proton for each electron. Hence the attraction is maximum and the ion is smallest among the given species. Greater this value, greater is the attraction and smaller is the size.įor example, in Na + ion, there is 1.1 proton for each electron. of protons (Z) to number of electrons correlates effective nuclear attraction. Related questions 2) Which of the following is the smallest in size? Note: It is not possible to get covalent and metallic radii for noble gases since they do not form bonds.Ībove fact is reflected in option "a". Hence neon's atomic radius must be much more than that of fluorine. The reported radii of noble gas elements are "van der Waals radii", which are 40% more than the actual atomic radii. PERIODIC TRENDS IN RADIUS OF ATOM AND IONĪdiChemistry IIT JEE Atomic radii of fluorine and neon in Angstrom units are respectively given by: Questions periodic trends | atomic radius | IIT JEE | NEET Overall the effective nuclear charge increases, there is a stronger attraction between nucleus and valence electrons, the nucleus can pull the electrons closer.


 0 kommentar(er)
0 kommentar(er)
